Customized applications for tracking quality-related events
We hear you!
➤ Off the shelf software is not customizable to your workflow.
➤ Your team resists submitting medication error reports due to poorly designed systems.
➤ You spend too much time and money analyzing data.
➤ You have low confidence that your efforts are reducing the risk of future errors and harm.
We design and deploy effective software. Start with AEMS, our core application for tracking errors, near-misses, unsafe conditions, and adverse events; supporting health systems, community pharmacies and retail pharmacies. Easily customize the user interface, analytical reports, and dictionaries to meet your unique needs. Easily track HIPAA incidents, investigations and conclusions.
Data Analytics
Our data scientists and database engineers will help you organize your quality-related event data (including errors, adverse events, near misses, unsafe conditions) and data from other specialized surveillance programs. We use standard analytical approaches and novel natural language processing analyses to maximize the value of the information your organization has already collected.
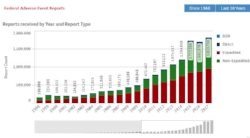
federal adverse event reporting system Database requests
FAERS - We will help you search, organize and analyze information from the FDA's primary portal for adverse event information about medications. The FAERS database includes approximately 15 million reports, with more than 8 million serious reports and 1.5 million death reports.
Freedom of Information Requests (FOI)
FOI - We will help you write and submit FOI requests to federal and state agencies to access those difficult to find public facts about medication and medical device related information. Then, we help you organize and analyze the results.
multi-media presentations
We've partnered with BeeryMedia, to produce customized multi-media presentations that help you tell your story about medication safety. Our content experts work closely with BeeryMedia's video production experts to tell informative and compelling stories used in training and development, teaching, marketing, documentaries, and public service announcements. We produce webinars for live or on-demand presentations covering a variety of topics including medication safety, drug interactions for patients, clinical research, research ethics, the responsible conduct of research and more.
Webinars and Distance Learning
We produce webinars for live or on-demand presentations covering a variety of topics including medication safety, drug interactions for patients, clinical research, research ethics, the responsible conduct of research and more.